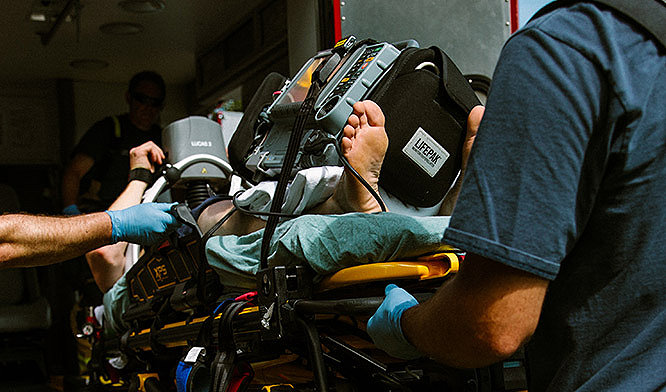
Emergency medical services
You work in one of the world’s most demanding, fast-paced environments. You need durable, powerful connected devices to help save lives and coordinate teams to deliver the highest quality of care.
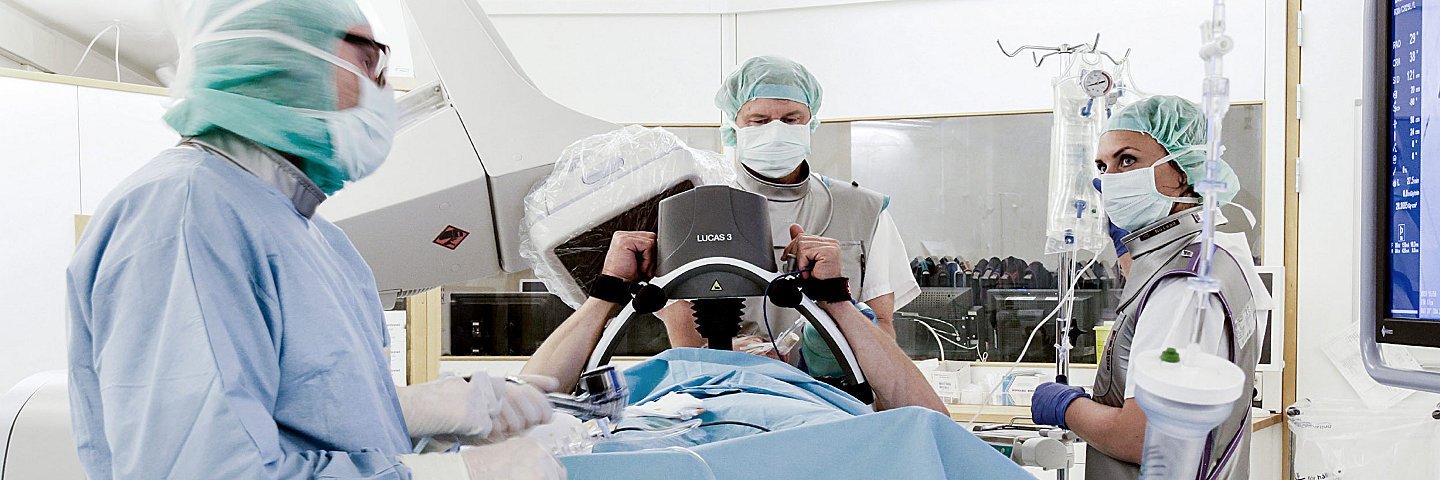
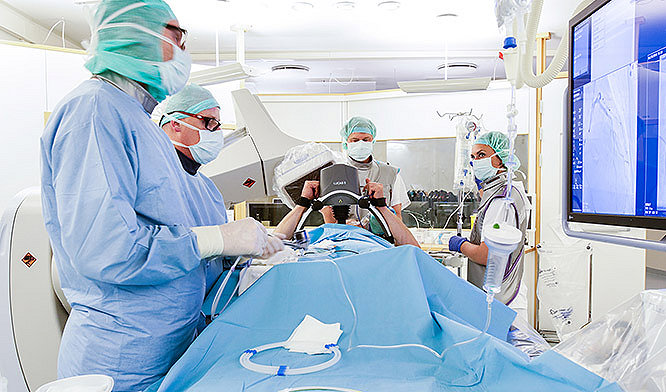
Hospitals
Equipment you can depend on in that moment when saving a life is the only thing that matters. Intelligent tools to monitor, document and review each code event to respond even better the next time.
Workplace and public safety
Sudden cardiac arrest (SCA) knows no boundaries. Our products and solutions can provide a critical resource to help a first responder—a colleague, a teacher or simply a passerby—save a life.
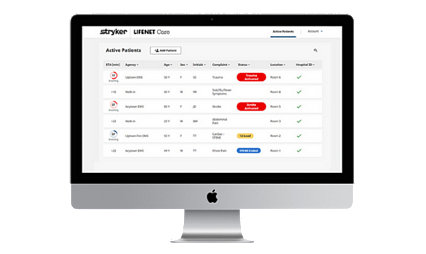
Our products share data, so you can focus on caring for patients.
Survivor stories: Derrick Mosley
After going on a morning run, Derrick began to feel some chest discomfort. His wife, Melissa, a healthcare professional trained in resuscitation, quickly recognized the warning signs of a sudden cardiac arrest. Luckily, an off-duty firefighter pulled over from traffic and began assisting. Soon EMS and Fire/Rescue units arrived to apply a LUCAS.
M0000016474 REV AA
3D biphasic defibrillation animation
This quick video simplifies the science of biphasic defibrillation and illustrates how escalating energy and pad placements impact shock success.
SERVICE AND SUPPORT
Robust support for your team
Technical support
We'll work with you to quickly assess your situation and find the best solution.
ProCare service plans
Focus on saving lives while extending the integrity of your devices with a ProCare service plan.
Product resources
Find a variety of documents including best practices, datasheets and clinical information for easy download.
Training and education
Discover webinars, online and in-person courses and other resources to strengthen your clinical education.
Connect with an expert
Have feedback about Stryker’s products and services? Visit our product experience page to connect with us.
M0000014366 REV AA
Featured products
Xpedition
Learn morePower-PRO 2
Learn moreLUCAS 3, v3.1 chest compression system
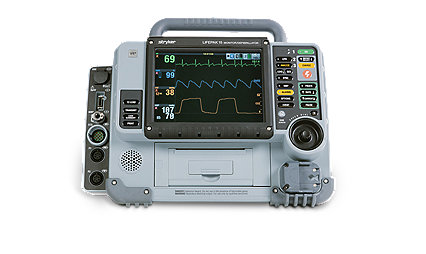
Learn moreLIFEPAK 15 V4+ monitor/defibrillator
Learn moreLIFENET Care
Learn morePower-LOAD
Our Power-LOAD cot fastener improves operator and patient safety by supporting the cot throughout the loading and unloading process.
Learn moreLIFEPAK CR2 defibrillator
Learn moreHeartSine samaritan PAD 450P
Learn moreAdditional resources
Product resources
Find a variety of documents including best practices, datasheets and clinical information for easy download.
Learn moreDisclosure and safety information
Learn moreLIFEPAK device registration
Learn moreTerms and conditions
Learn moreM0000010087 REV AB